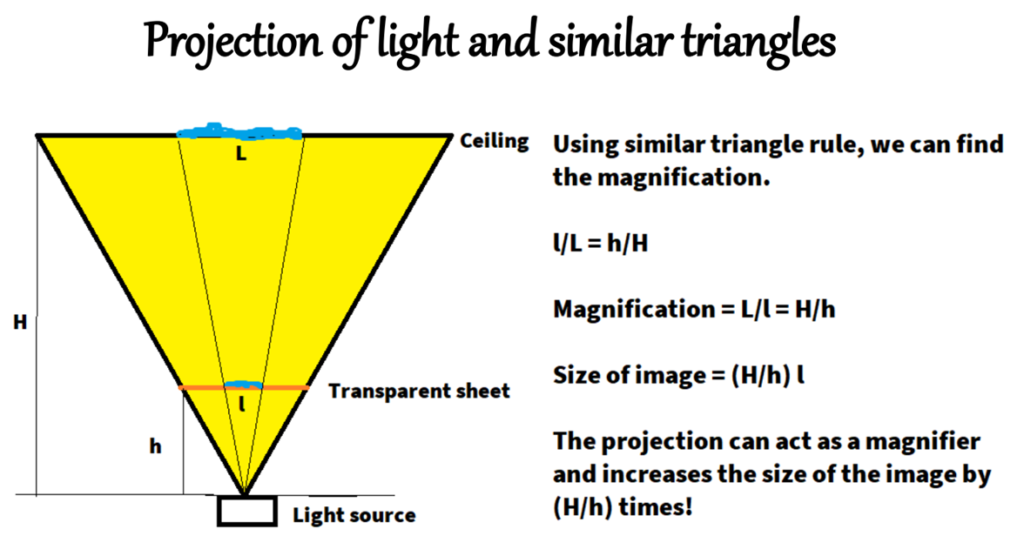
It is of common knowledge that oil and water do not mix together, but can we see them push each other away and clearly form an immiscibility gap? Yes we can, and that too in a very lucid way! All we need is our mobile torch, a dark room and a transparent glass table/sheet with stand. In a dark room, the light is passed from below the transparent glass and projected onto the room ceiling. Diverging light rays from the source form two similar cones; the smaller one with the base as the glass surface and the magnified one on the top with base as the room ceiling. A simple schematic is given in Fig.1.
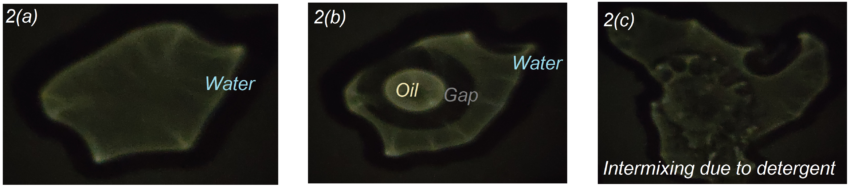
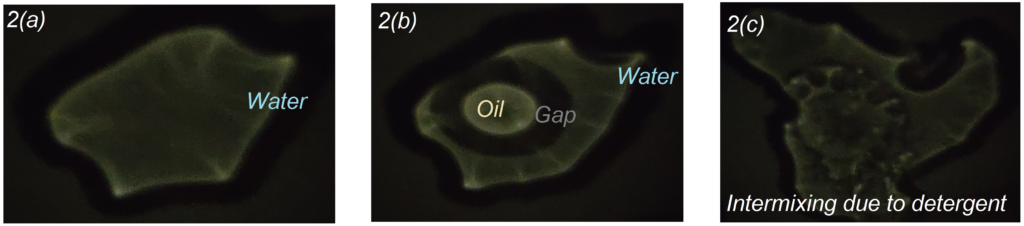
When few drops of water is poured on top of the glass and we look at the ceiling, we see a bright projection of the water droplet magnified easily by 20-50 times as given below in Fig2(a). When a few drops of oil is poured near it, we can see how water and oil push each other away and form a clear gap in between. The gap region appears dark and the edges appear bright because the light diffracts from rarer to denser medium, thereby making the edges shine brighter and the gap more darker and vividly visible. We can also see how oil forms a near perfect circle, making the best attempt to reduce the surface area, as given in Fig.2(b). Now, when we add some detergent to the oil, we can see how the oil sphere bursts open and readily mixes with water, as if the nucleus of an amoeba has been burst open and all the content is spreading into the cytoplasm, as shown in Fig 2(c).
This visual light show in the ceiling can not only promote the cause and joy of chemistry, but also act as an empirical base (visible Pratyaksh Praman) onto which concepts dealing with the invisible atoms, their electronegativity, types of bond formation, polar and non-polar bonds, hydrophobic/hydrophilic/amphiphilic substances, surface tension, solubility and miscibility etc. can be discussed in detail. The same setup can be used to illuminate many more surface phenomena like kinetics of dissolution (between sugar and salt), effect of particle size on dissolution, capillary action, turbulent and lamellar flow, and many more which the creative chemistry educators can explore, imagine and improvise.
Demonstrations of above activity:
1) Demo with an empty jam bottle: https://www.youtube.com/watch?v=8Em1lNGhHO8
2) Online hands-on activity session to the students of Shiv Nadar School and Indian Youth Nuclear Society: https://www.youtube.com/watch?v=yS6FED8fJAo