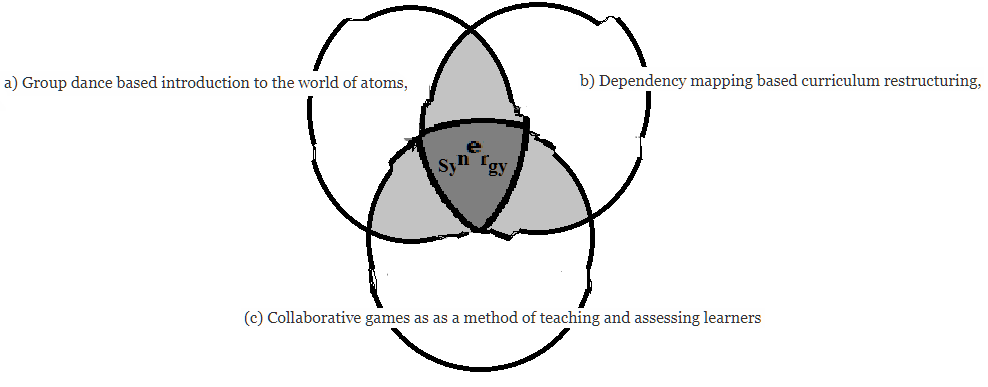
Asan Vigyan is an initiative to try create and evolve new methods for teaching and learning science in an enjoyable and effective way. Our objective here was to see what happens if we synergistically combine
a) Group dance based introduction to the world of atoms,
b) Dependency mapping based curriculum restructuring, and
(c) Collaborative games as as a method of teaching and assessing learners the ABC of chemistry.
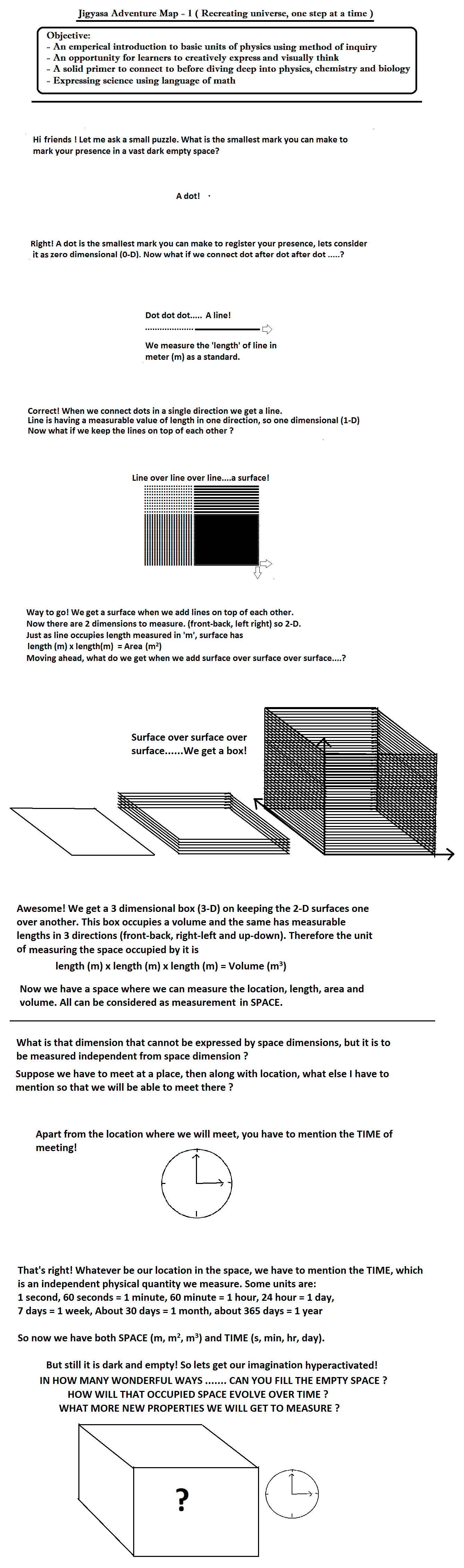
The first chapter in 10th std science is the ‘school of atoms’ or ‘nature of matter’. As the formal syllabus follows historical lineage of concepts, Jigyasa is here to supplement it with contextual lineage of inter dependent concepts. The classroom activity designed as Jigyasa Adventure Map starts asking about the smallest mark that can be made to introduce space and then time is introduced as a unit that space cannot measure. It ends in the form of another fresh initiative as in to know in how many ways we can fill an empty space-time[1].
________________________________________________________________________________________________________
The second series starts with broadly classifying ‘whatever that occupies space and time’ as ‘matter’ and ‘light’ and then it ventures into discussing the first fundamental property of matter, mass. It proceeds with explaining how massive particles are made up of smaller unit called ‘ATOMS’. Just as how dots can fill up space, or how droplets fill ocean, bricks make the wall etc, thus amongst the smallest blocks of matter were termed as atoms.
Learning of ‘states of matter’ can be actively and collaboratively done as ‘Atom Dance’, a group dance activity with every student representing an atom or group of atoms, together they change phase from solid to liquid to gas, on changing the ‘temperature’, in our case by changing the drum beat frequency that guides the movement of students who are physically simulating the atoms [2].

Once the basics of matter and a feel of how solids, liquids and gases are made up of atoms is understood, we can proceed into the complexities of chemistry. i.e. types of atoms–>their constituents–> charged particles –>electricity and magnetism–>protons, neutrons and electrons–>light as photons–>quantization of energy levels–>valence electrons, valency and oxidation state–> periodicity–>metals, non metals and metalloids–> Thermodynamics of chemistry i.e. how the fundamental particles love to loose some energy and go to lower energy state if given a chance–>
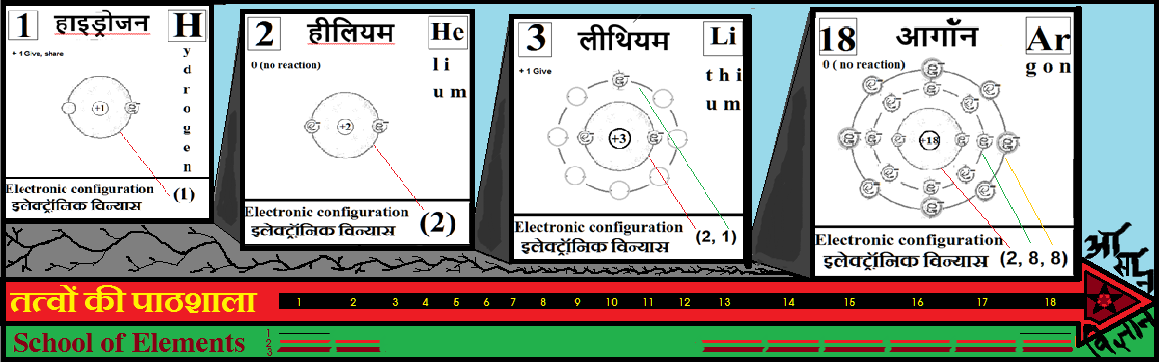
Noble gases–> Octet rule–> In this process once in a while it is a good idea to brush up the basics of at least the first 18 elements of the periodic table.
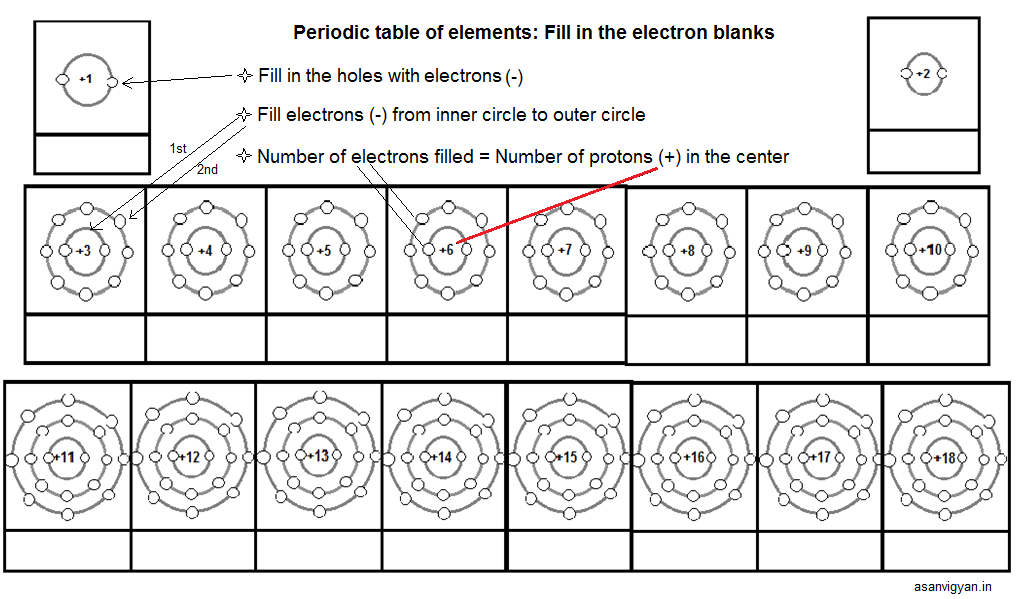
Basics can be done teaching them how to up their ‘periodic table: fill in the electron blanks worksheet’
_________________________________________________________________________________________________________
For the answers one can refer to the periodic cards:
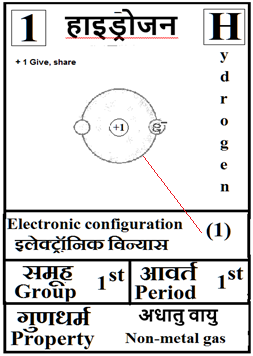
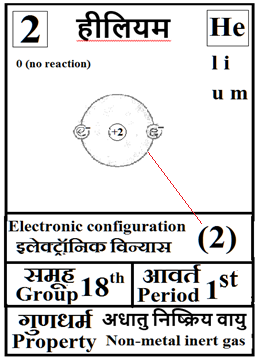
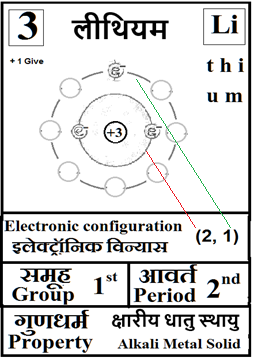
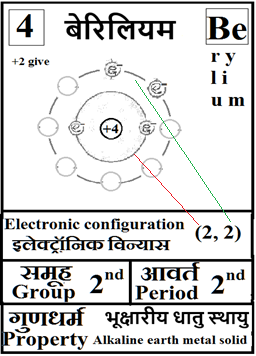
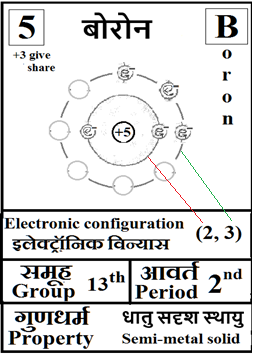
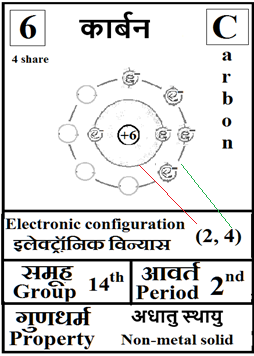
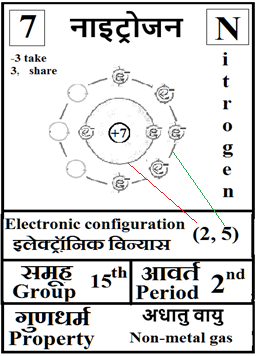
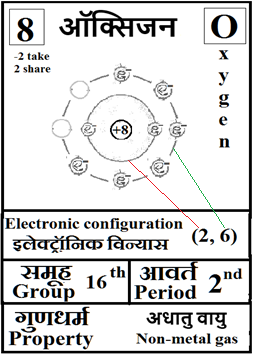
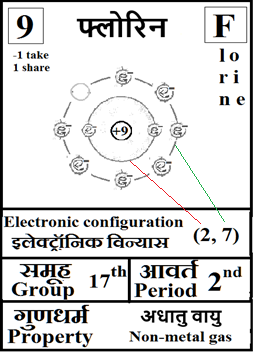
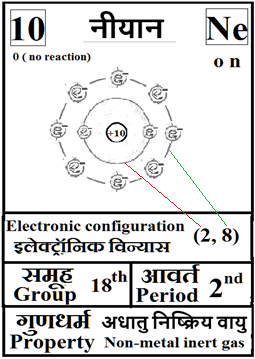
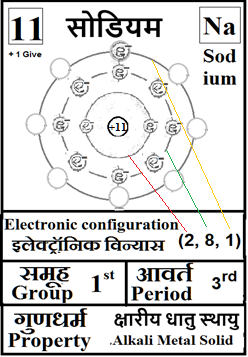
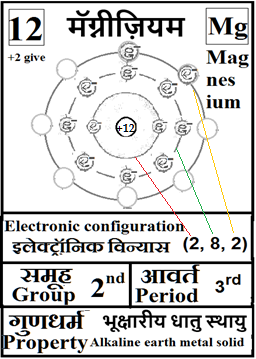
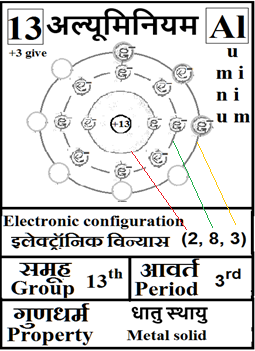

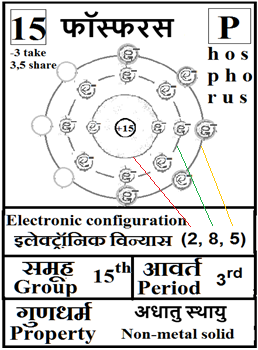
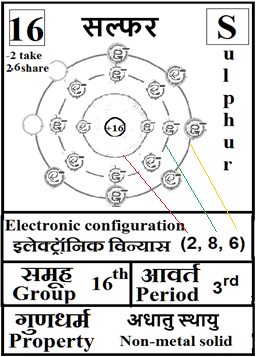
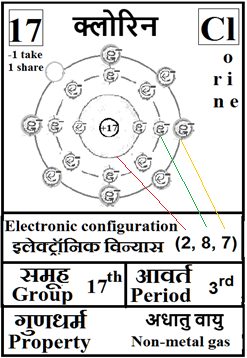
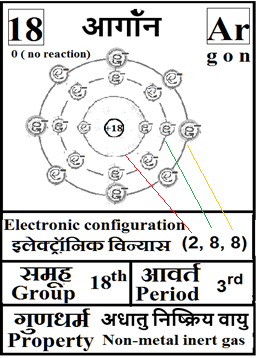
________________________________________________________________________________________________________
Progress in learning can be periodically assessed by playing atomic card memory game.
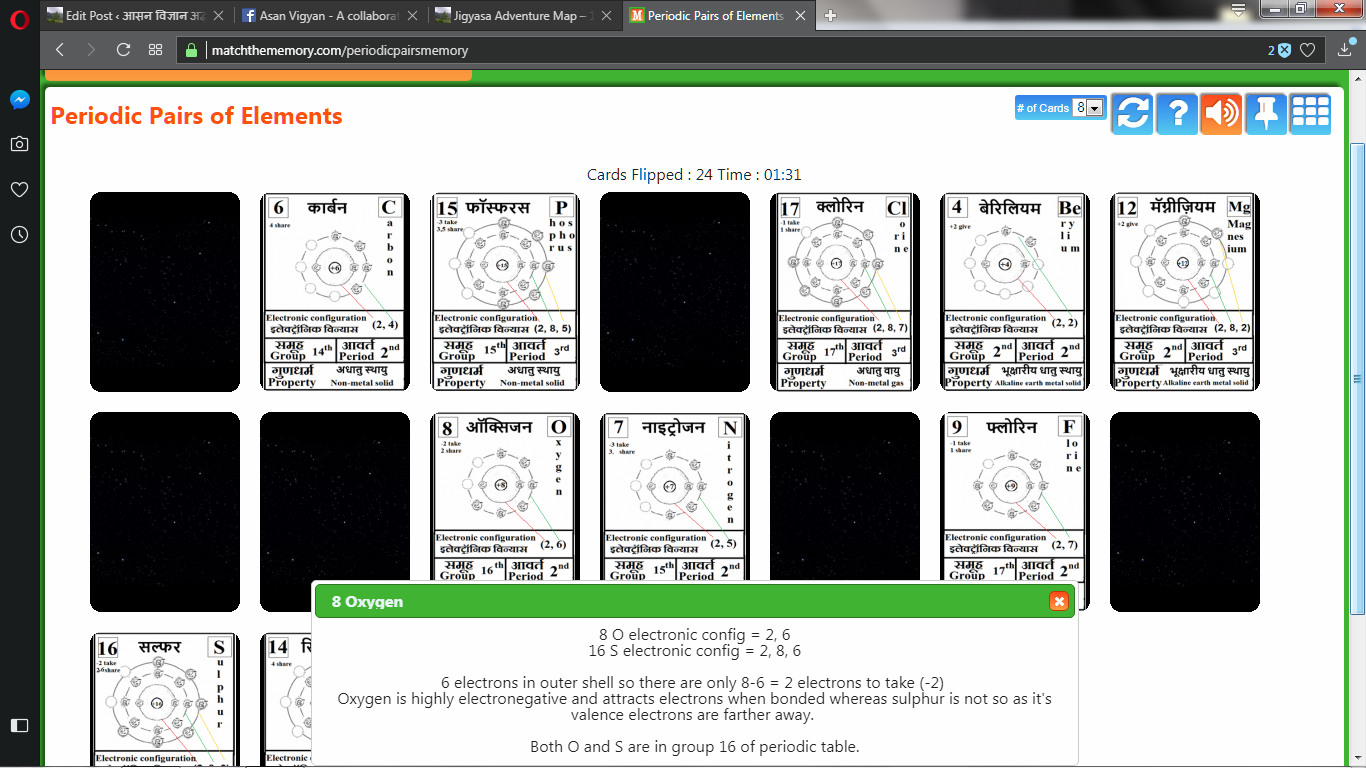
Login into: https://matchthememory.com/periodicpairsmemory
From this game we can get data about the players ‘total time taken (s)’ and ‘total number of flips taken’.
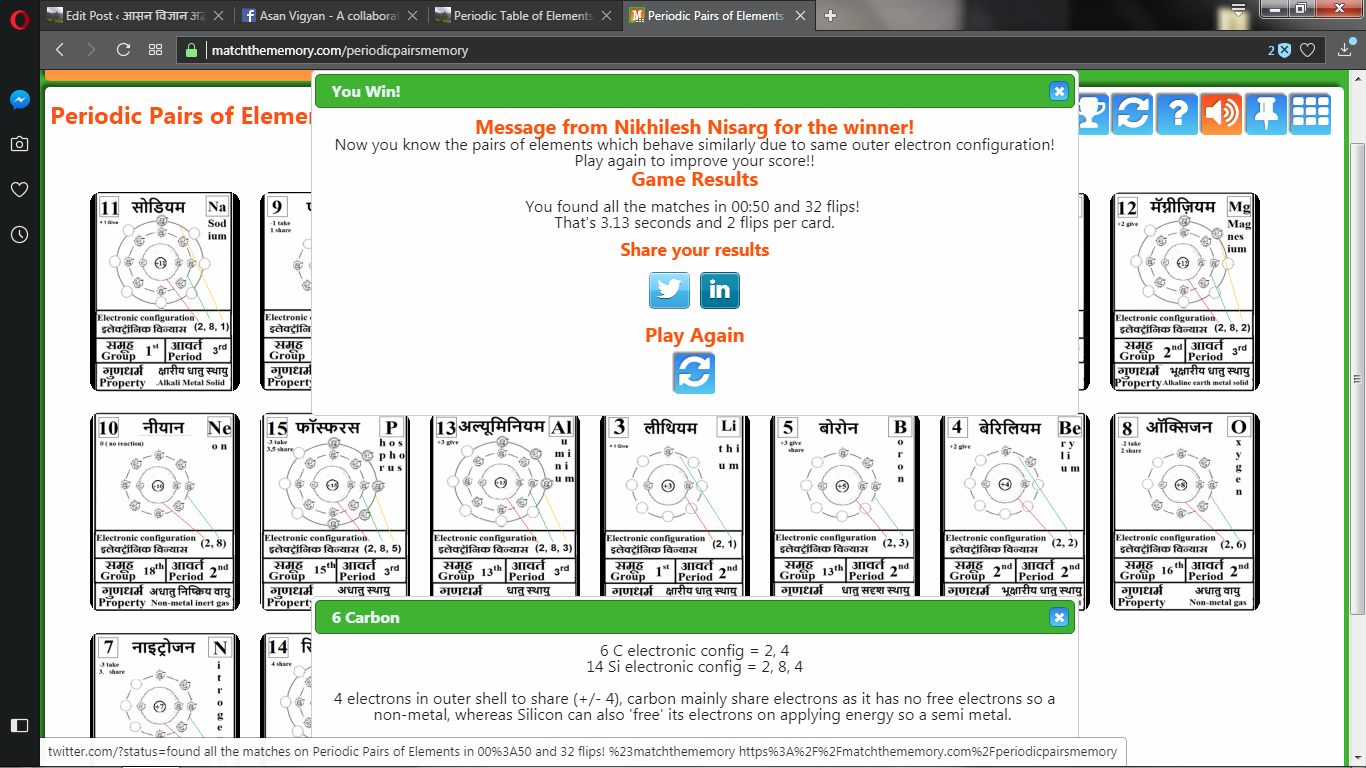
Every player gets to play as many times as they want to. After the game is over they can roll their mouse over the cards and see matched pairs and see how valency decides the chemistry of periodic pairs of elements.
Data collection has been initiated, starting with 17 students of Samta Hindi Vidyalaya, Turbhe, Navi Mumbai.
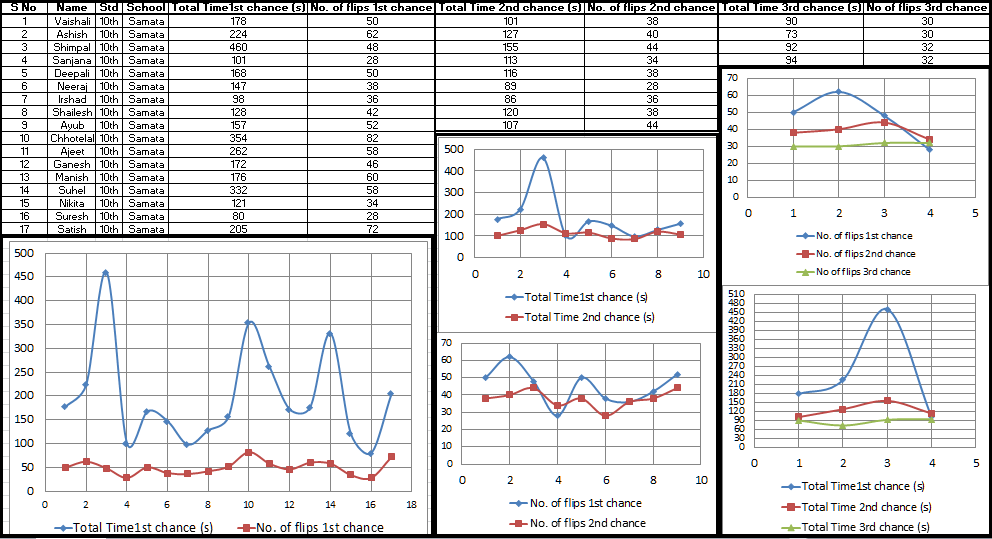
The improvement seen in the performance is evident with overall reduction in both number of flips and time taken over repeated chances.
Passing Conditions:
- The learners will pass the course only if they fill up the electrons table consistently 3 times.
- In terms of Periodic pairs memory, they also have to more than 5 times get total time taken <60 s and total flips <40.
This method of testing can be used to supplement formal exam system, and also to implement evaluation or assessment system that are
- self-paced
- collaborative
- mastery oriented
- gamified
- freely available
Later the chapter can continue in the direction of: Formation of compounds–> chemical bonds–>Covalent, Metallic, Ionic bonds—> Dipoles —>Chemical reactions—>……to be continued…
_________________________________________________________________________________________________________
Here we wish to raise a few question,
Are competitive exams necessary ?
Will a competitive model of school assessment and performance appraisal in offices serve the dreams of the next billion in the 21st century ?
If something is considered so important that it is a part of ‘curriculum’, and of course we are taking away the precious time of a little child to tell them about the world we are living in, shouldn’t we make sure that each and every child gets to learn it completely and attain mastery ?
Can they take their levels up in their own pace ? How to synergize learning and promote collaboration ?
_________________________________________________________________________________________________________

