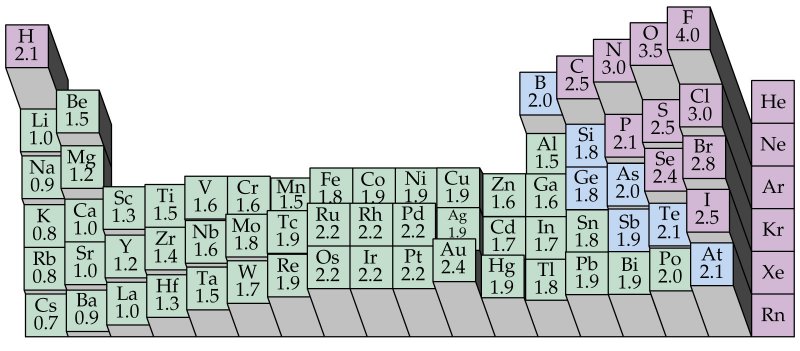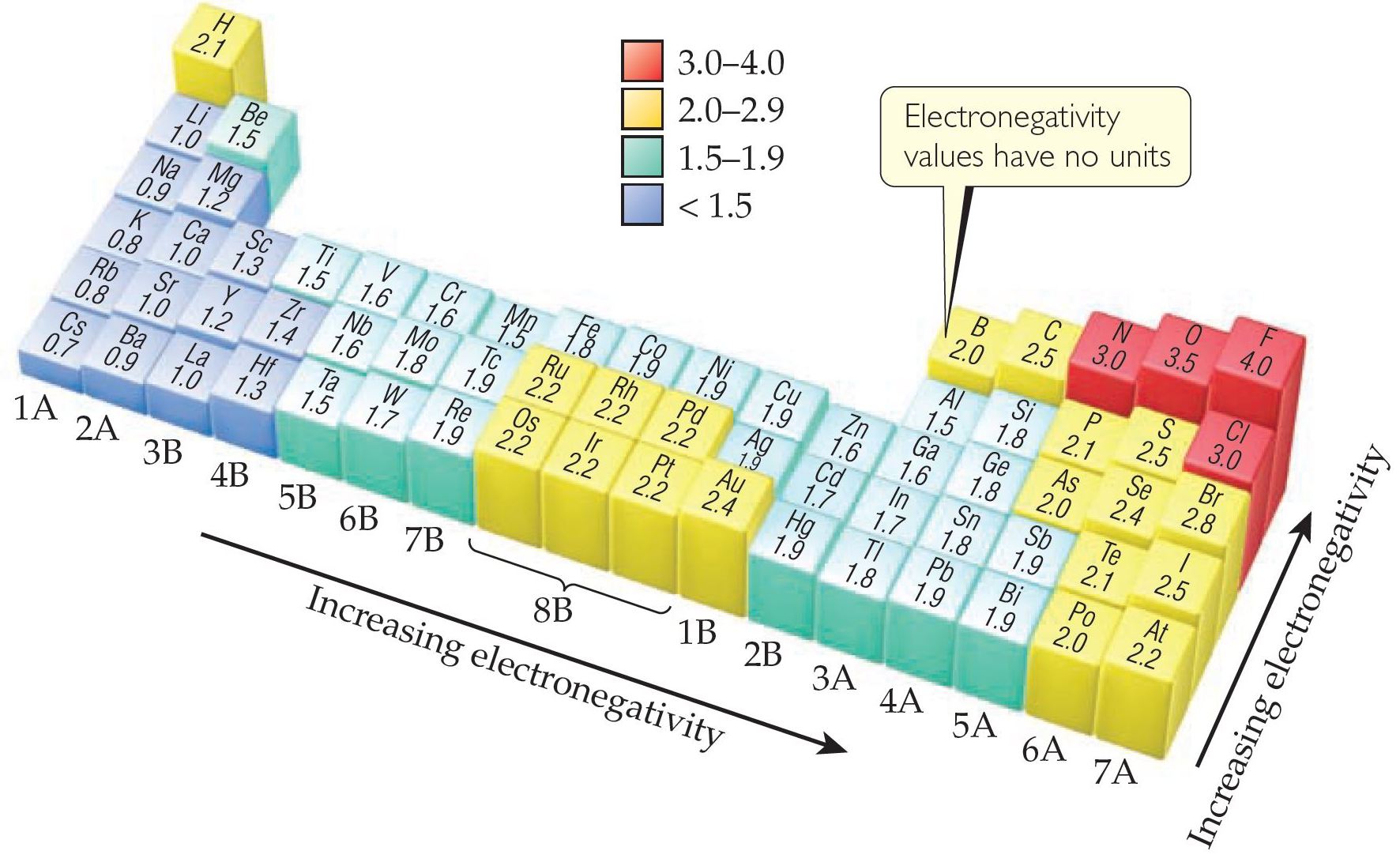
(https://www.tollebild.com/bilden/electron-negativity-chart-1b.html)
In this vast wonderful and confusing forest called chemistry, if there is any map that can help the explorers navigate towards the treasures of nature, then arguably it has to be the electronegativity map.
This map can be understood even without going into the finer details of electronic structure, but on going into the finer details we can appreciate why this map has this specific topography; and what do these peaks, planes and valleys actually intend to say.
So a small QnA style discussion of electronegativity:
Q) What are the pre-requisites to understand electronegativity ?
A) The pre-requisites are:
1) Matter in this world is made up of very very small atoms. Atoms are made up of protons (+ charge) and neutrons (0 charge) in the centre with electrons ( -ive charge) doing their usual & sometimes unusual quantum stuff bound near the nucleus, and they are of the same number as that of protons.
2) There are different kinds of atoms depending on their number of protons in the center,( AKA Atomic Number), and they are arranged in the periodic table of elements.
3) Since electrons repel each other, they cannot clump the way protons get clumped (protons get help of the neutrons to together invoke the Strong Force!). Also they need some space to roam about and spin.
So the electrons push each other around and form shell like structure, that is layering one over the other a little like onion peels.
4) In general atoms are not very satisfied with their fate of electronic configuration; so they give, take or share electrons with themselves and each other to form compounds. This whole giving taking and sharing of electrons which make two atoms stick together is called as the bond….chemical bond.
Q) Hmm…So…what does it has to do with electronegativity ?
A) Since all elements don’t behave the same way, all bonds between the elements are also not the same! In the periodic table of electronegativity as given above, those elements in the valleys with low value of electronegativity prefer to give away their electrons, and those in the peaks loves to pull the electrons towards itself. Of course there are those in the middle which wisely decides to complement their partner.
Q) What does electronegativity of an atom depend upon ?
Given below is the table of atomic size (Taken from: http://www.crystalmaker.com/support/tutorials/atomic-radii/)


On comparing the two we see that elements on the top right are small with higher electronegativity, especially F, O, N and Cl. In a given row, as the atomic number increases the electronic outer shell shrinks due to more pull from the increased central charge. As we move down a column, we see that new shells are added so outer electrons are farther away from the pull of their central positive charge.
Can you guess why inert elements in the last row, that is atoms with completely filled outer structure have no electronegativity ? Because they don’t react, or participate in the electron marketplace.
Q) What is the consequence of electronegativity in chemical bond?
A) Electronegativity arguably governs the nature of chemical bond.
Bond between elements with very low electronegativity (Na and Na): So both want to give their electrons so they form metallic bond.
Bonds between elements with one having very low electronegativity and other with high electronegativity (Na and Cl): In that case atom with very low electronegativity will give its electron completely to become a cation (+ ion, Na+) and the one with high electronegativity will take that electron to become anion (-ion, Cl-). These opposite charged ions are bound by electrostatic attraction (Na+ and Cl-) also known as ionic bond.
Bonds between atoms of same element with high electronegativity (O and O): They both are pulling electrons with equal strength so the tug of electron war is a tie and they have to share electrons. They form covalent bonds.
Bonds between atoms when electronegativity difference is high but not too high: They lie somewhere between pure ionic and pure covalent and can be termed as polar covalent bond.
Bonds between atoms when electronegativity difference is low: They too lie in between pure covalent and pure ionic but much closer to the equal sharing side, forming non polar covalent bond.
With this basic info covered, we can then dwell into the formation of compounds.